In quantum mechanics photons exist in a cloud-shaped area outside the nucleus. It has changed the way we look at an atom If we look at the shell model we get a very 2d view the make us realize that the location of an electron is not limited upto their orbits but rather there are only 90 chances of electrons to be detected in orbits.

What Is Quantum Mechanics Quantum Physics Defined Explained Live Science
How did quantum mechanics change our understanding of atoms.

. The big change in understanding that happened with Quantum Physics is the idea that the universe is random rather than clockwork at its lowest level. All this was and is still really confusing to any common person. Answers is the place to go to get the answers you need and to ask the questions you want.
In quantum mechanics electrons exist in a cloud-shaped area outside the nucleus. It has changed our basic understanding about stability and location of an electron in an atom. Quantum theory suggested that an electron can be a particle as well as a wave at same time.
IF we look at the shell model we get a very 2d view T he quantum mechanics make us realize there is a lot more to realizing the location of an electron. In quantum mechanics photons are a part of the nucleus along with electrons. Heisenbergs Equation where x is the position p is the momentum and h cross is the Plancks constant divided by 2pi.
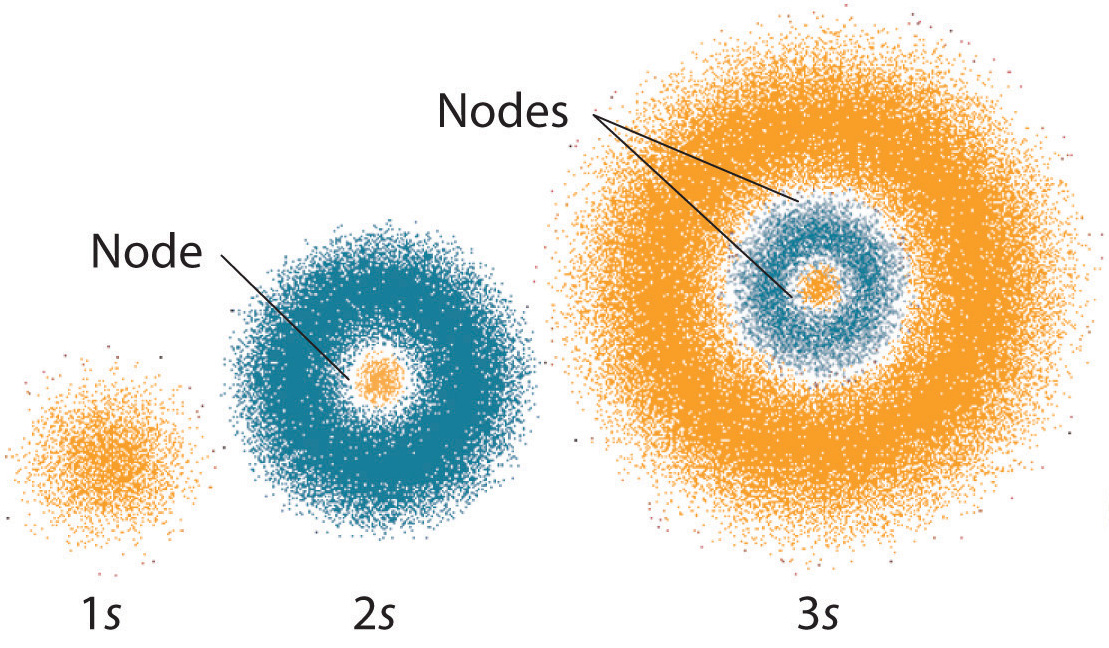
The Quantum Mechanical Model Of The Atom Article Khan Academy

Physical Science Quantum Mechanics Britannica

0 Comments